Real-time control over a chemical reaction network by light
02.09.2024
 |
|
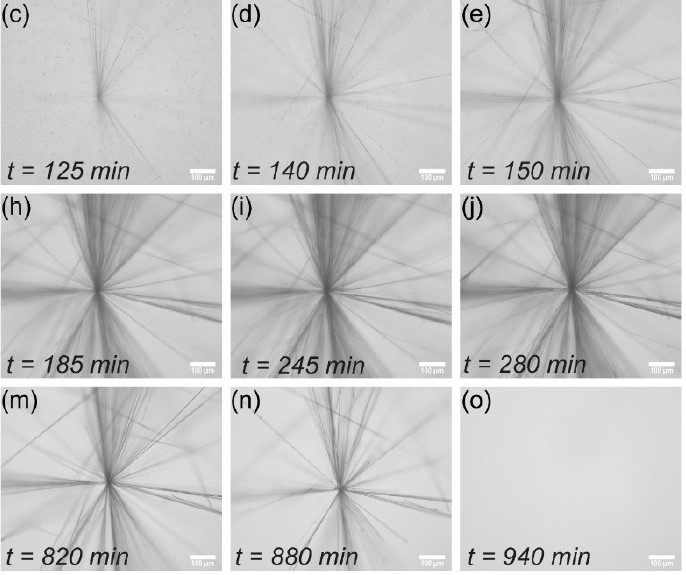
Microscopy images of the reaction network producing fibers in a star-like shape. Credit: Jorge S. Valera. |
- IMDEA Nanociencia researchers control the activation and deactivation of a molecule's self-assembly through light, using two different wavelengths.
- The control of the reaction is carried out in a dissipative manner, through the continuous consumption of fuels.
- The control of self-assembly in networks of artificial chemical reactions provides important information that can be used to understand their analogue in nature: actin filaments, microtubules, etc.
| Tweet |
Madrid, 2nd September, 2024. Self-assembled molecules are responsible for important cellular processes. Self-assembled structures such as microtubules or actin filaments are key to cell motility: change of shape, division or extension of membranes. These self-assembled entities have the peculiarity of being formed temporarily, since they require energy consumption. Inspired by nature, there is currently an active area of research that attempts to replicate this process of self-assembly artificially, using the so-called chemical reaction networks.
The control of self-assembly by means of chemical reaction networks is based on the activation of a monomer prone to self-assembly, which is then deactivated. In this way, the self-assembled structure requires a continuous energy consumption to perpetuate itself. From a chemical point of view, this energy is provided by a "fuel", a chemical reagent. Depending on the availability of that energy source, the self-assembly process occurs or not. Traditionally, highly reactive fuels have been used to carry out the activation, with little control over the deactivation process. This also implies that the activation and deactivation fuels tend to react with each other, making artificial dissipative self-assembly processes ineffective. In nature, these two processes are controlled by catalysts, which increases their efficiency. Thus, the introduction of catalysts in these processes and the control of their activity by external stimuli such as light are highly desirable, since they can limit part of these problems.
In the Systems Chemistry Group, led by Dr. Thomas Hermans at IMDEA Nanociencia institute, dissipative self-assembly processes, or how to organize molecules to form organized structures by consuming fuel, are studied. In a previous work, the researchers have already investigated how to use a photocatalyst, a light-activatable substance that increases the speed of the reaction, to carry out self-assembly cycles. Now, in a recent work, two catalytic processes modulated by light have been used to control activation and deactivation, respectively, and which, using two wavelengths, have allowed better control over the life of the self-assembled species.
By using a photocatalyst in the first part of the cycle, the researchers were able to store the fuel in the system until they wanted to start the reaction. Specifically, the monomer was irradiated with blue light, thus initiating the process of transient self-assembly of the aldehyde through oxygen consumption. Then, the deactivation of the self-assembled structure by radiating with ultraviolet light began. This light allowed a second fuel (format) to be released that launched the catalytic cycle of deactivation. The main novelty of the work is that it has been possible to have a transient and autonomous self-assembly process that can be activated with light, as well as a certain control over the life of the self-assembled species through the irradiation of light.
In this way, activating and deactivating the self-assembled structure with lights of two different wavelengths is a great advantage, because it limits the reactivity between fuels and allows better control of the deactivation of the self-assembled molecule. Dr. Jorge S. Valera, lead author of the work, comments: "We have a very complex system of various molecules, in which it is very difficult to control individually all the processes that are carried out, and globally what we observe is that we are able to control the life of the self-assembled structure by coupling two catalytic processes activated with lights of different wavelengths."
These results, published in Angewandte Chemie, are framed within so-called "life-like" materials that try to imitate the behavior of nature, being able to take signals from the environment and process them, like a "chemical software". By controlling the activation and deactivation of the self-assembled species in a dissipative manner, behaviors analogous to those observed in cells can be observed and studied: oscillations when rapidly forming/destroying that structure, coupling two activation/deactivation processes and subjecting it to a rupture, and forcing out-of-equilibrium conditions in which the system has to recover very quickly. But studying these networks of chemical reactions can also lead to materials with novel properties, such as polymers that regenerate, and you could control when they self-repair, or how long they use.
This work is a research result carried out at the University of Strasbourg and IMDEA Nanociencia, and has been partially funded by the European Research Council (ERC 757910), the Marie Sklodowska-Curie Actions (812868) and the Severo Ochoa Seal of Excellence to IMDEA Nanociencia (CEX2020-001039-S).
Reference:
Jorge S. Valera, Álvaro López-Acosta and Thomas M. Hermans. Photoinitiated transient self-assembly in a catalytically driven chemical reaction cycle. Angewandte Chemie Int. Ed. 2024. DOI: 10.1002/anie.202406931
![]() https://repositorio.imdeananociencia.org/handle/20.500.12614/3681
https://repositorio.imdeananociencia.org/handle/20.500.12614/3681
Contact:
Thomas M. Hermans
This email address is being protected from spambots. You need JavaScript enabled to view it.
https://www.imdeananociencia.org/systems-chemistry-laboratory/home
https://x.com/Hermanslab
Oficina de Divulgación y Comunicación en IMDEA Nanociencia
divulgacion.nanociencia [at]imdea.org
Twitter: @imdea_nano
Facebook: @imdeananociencia
Instagram: @imdeananociencia
Source: IMDEA Nanociencia.